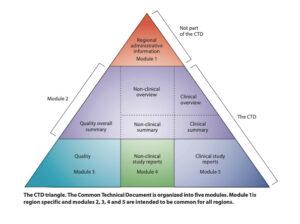
Obtaining marketing authorization (MA, AIC in Italy) is a process that requires a rigorous application of reference regulations, first of all Directive 2001/83, and the presentation of the required documentation, through the production of the electronic Common Technical Document (eCTD).
After having identified with our client the regulatory application that allows us to choose the best type of registration to carry out, we then:
- Prepare the eCTD using a validated platform, following the International Council for Harmonization (ICH) guidelines,
- Submit to the regulatory authorities, and manage the institutional relations.
SCF provides the following services:
- Preparation of printed matter (summary of product characteristics-RCP, labels and package leaflets) according to the current guidelines,
- Support for Italian and EU registration of medicines (centralized, decentralized, national and mutual recognition procedures),
- Management of the variations and conversions of CTD into eCTD,
- Registration of medicine brokers and distributors at the Ministry of Health,
- Evaluation of regulatory conformity of the material for medical-scientific information.
Moreover, thanks to our partnership with CRO UNIFARM, we help our clients conducting interventional, observational and bioequivalence studies, as well as qualitative-quantitative analyses according to GLP (Good Laboratory Practice).
